Abstract

Background: Anti-CD30 chimeric antigen receptor (CD30.CAR) T-cell therapy has been studied in heavily pretreated relapsed/refractory (r/r) classic Hodgkin lymphoma (cHL) patients with an objective response rate (ORR) of 62% and complete response (CR) rate of 51% (Ramos et al, JCO, 2020). Despite high response rates, disease progression has been observed, with a 1-year PFS of 36%. The anti-PD-1 antibodies nivolumab and pembrolizumab have both been studied in phase II studies in patients with r/r cHL with ORR ranging between 65-87% and median progression free survival (PFS) ranging from 13-15 months. Limited data is available regarding the clinical benefit of anti-PD-1 therapies following CD30.CAR-T cell therapy and the potential mechanism of response in patients with prior anti-PD-1 exposure.
Methods: We followed and analyzed the clinical outcome of 10 patients with r/r HL who progressed after CD30.CAR-T cell therapy at the University of North Carolina at Chapel Hill and subsequently received anti-PD-1 therapy to treat relapsed disease. Clinical responses in follow up were determined per local radiologist assessment. Expansion and persistence of CD30.CAR-T cells in vivo was determined by quantitative PCR from peripheral blood samples.
Results: Between October 2016 and November 2019, patients were treated with either CD30.CAR-T cells (9 patients) or CD30.CCR4.CAR-T cells (1 patient) on one of three clinical trials (NCT02690545, NCT02663297, NCT03602157). The median age of patients was 30 years (range, 24-51 years). The median number of prior lines of therapy was 8 (range, 5-16). Eight patients had a prior autologous stem cell transplant, and six patients had a prior allogeneic stem cell transplant. The best response to CAR-T cell therapy was a CR in 5 patients, partial response (PR) in 1 patient, and stable/progressive disease in 4 patients.
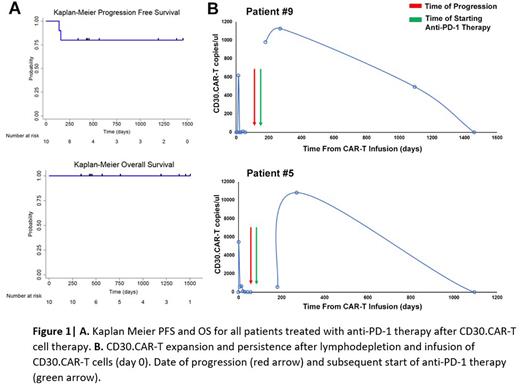
At the time of progression following CD30.CAR-T cell therapy, patients were offered standard of care anti-PD-1 therapy. Eight patients were treated with nivolumab, and 2 patients were treated with pembrolizumab based on decision of the treating physician. The median time from CAR-T cell therapy to anti-PD-1 therapy was 171 days (range, 56-858 days). Seven of the 10 patients had prior anti-PD-1 exposure before CAR-T cell therapy. All 10 patients had an objective response to anti-PD-1 therapy after CAR-T cells, with a best response of CR in 7 patients and PR in 3 patients. Four of the 6 patients who had a PR to anti-PD-1 therapy prior to CAR-T cell treatment, achieved CR with anti-PD-1 re-challenge afterwards. At the time of the last follow up, 8 patients remain in an ongoing response, lasting over 1 year in 7 and over 3 years in 3 patients. The median PFS was not reached in this cohort (Fig 1A). Five patients stopped anti-PD-1 therapy after CAR-T cell therapy (4 related to toxicity of therapy, 1 for ongoing durable response). At the time of data collection, with a median follow up of 3.6 years (range, 1.7-4.7 years), all patients were still alive.
Longitudinal peripheral blood samples were available in 3 of the 10 patients. All 3 patients with longitudinal samples demonstrated expansion and/or persistence of CD30.CAR-T cells following anti-PD-1 therapy (Fig 1B). Patient #9 and #5 had impressive expansion of CD30.CAR-T cell signals shortly after start of anti-PD-1 therapy. Patient #1 had detectable PCR signals in the peripheral blood until at least 1 year after CD30.CAR-T cell infusion; however, sample timepoints were not available to assess for expansion of CD30.CAR-T cell signals shortly after anti-PD-1 therapy.
Conclusion: To our knowledge, this is the largest cohort of patients with r/r cHL who have received anti-PD-1 therapies after CD30.CAR-T cell treatment. Clinical efficacy appears strong, with high response rates and durable remissions observed even if most patients had prior exposure to anti-PD-1 therapy. All patients with no prior anti-PD-1 exposure experienced durable remissions. This study is the first to describe CD30.CAR-T expansion following anti-PD-1 therapy. While further analysis is warranted to assess for CD30.CAR-T functionality after anti-PD-1 exposure, our data suggest that anti-PD-1 therapy may rescue CD30.CAR T cells, supporting further investigations in patients with cHL.
Disclosures
Voorhees:Incyte: Research Funding; AstraZeneca: Research Funding. Morrison:Vesselon: Consultancy. Park:G1 Therapeutics: Consultancy; Epizyme: Membership on an entity's Board of Directors or advisory committees; Teva: Consultancy; Rafael Pharma: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Research Funding; Gilead: Speakers Bureau; Seattle Genetics: Research Funding, Speakers Bureau; Morphosys: Membership on an entity's Board of Directors or advisory committees. Dotti:Catamaran: Consultancy; Bellicum Pharmaceuticals: Consultancy. Serody:STING activation: Patents & Royalties: provisional patent to enhance CAR therapy for solid tumors/ provisional patent for the use of ILC2 cells to treat or prevent GvHD. Savoldo:Tessa Therapeutics: Consultancy. Grover:ADC: Other: Advisory Board; Genentech: Research Funding; Kite: Other: Advisory Board; Novartis: Consultancy; Tessa Therapeutics: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal